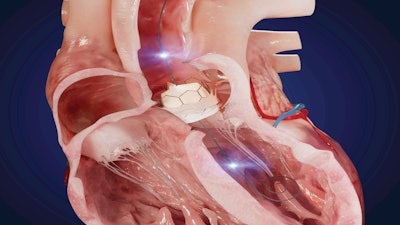
Xenter announced the recent unveiling of its dual sensor investigational guidewire for TAVR procedures at the Transcatheter Cardiovascular Therapeutics (TCT) 2023 conference in San Francisco, CA. Xenter introduced the wireless guidewire designed for use during transcatheter aortic valve replacement (TAVR) procedures that aims to collect an extensive range of crucial real-time data points to serve as an aortic regurgitation measurement (xAR) and clinical decision support tool.
The guidewire's functionality is designed to allow for comprehensive data collection and analysis and facilitates data necessary for novel Artificial Intelligence (AI) decision making tools. The goal is to enable better-informed clinical decisions and improve patient outcomes. The guidewire is designed to operate seamlessly within a proprietary wireless ecosystem in the Cardiac Catheterization Lab making real-time data and analysis more accessible than ever.
“An excellent example of real-time PI data collection is the TAVR SmartWire,” said William A. Gray, M.D., System Chief of Cardiovascular Medicine at Mainline Healthcare in Philadelphia. “TAVR SmartWire will enable the real-time measurement of aortic and left ventricular pressure information in a single workhorse device that can be used to develop a measurement of Aortic Regurgitation (AR). We plan to clinically evaluate TAVR SmartWire and provide physicians with a real-time AR measurement called xAR.”