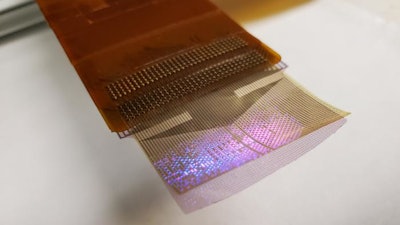
A thin film that combines an electrode grid and LEDs can both track and produce a visual representation of the brain’s activity in real-time during surgery–a huge improvement over the current state of the art. The device is designed to provide neurosurgeons visual information about a patient’s brain to monitor brain states during surgical interventions to remove brain lesions including tumors and epileptic tissue.
Each LED in the device mirrors the activity of a few thousand neurons. In a series of proof-of-concept experiments in rodents and large non-primate mammals, researchers showed that the device can effectively track and display neural activity in the brain corresponding to different areas of the body. In this case, the LEDs developed by the team light up red in the areas that need to be removed by the surgeon. Surrounding areas that control critical functions and should be avoided show up in green.
The study also showed that the device can visualize the onset and map the propagation of an epileptic seizure on the surface of the brain. This would allow physicians to isolate the ‘nodes’ of the brain that are involved in epilepsy. It also would allow physicians to deliver necessary treatment by removing tissue or by using electrical pulses to stimulate the brain.
“Neurosurgeons could see and stop a seizure before it spreads, view what brain areas are involved in different cognitive processes, and visualize the functional extent of tumor spread. This work will provide a powerful tool for the difficult task of removing a tumor from the most sensitive brain areas,” said Daniel Cleary, one of the study’s coauthors, a neurosurgeon and assistant professor at Oregon Health and Science University. Cleary was a medical resident and a postdoctoral researcher at the University of California San Diego.
The device was conceived and developed by a team of engineers and physicians from University of California San Diego and Massachusetts General Hospital (MGH) and was led by Shadi Dayeh, the paper’s corresponding author and a professor in the Department of Electrical and Computer Engineering at UC San Diego. The team describes their work in the April 24 issue of the journal Science Translational Medicine.
During brain surgery, physicians need to map brain function to define which areas of the organ control critical functions and can’t be removed. Currently, neurosurgeons work with a team of electrophysiologists during the procedure. But that team and their monitoring equipment are located in a different part of the operating room. Brain areas that need to be protected and those that need to be operated on are either marked by electrophysiologists on a paper that is brought to the surgeon or communicated verbally to the surgeon, who then places sterile papers on the brain surface to mark these regions. “Both are inefficient ways of communicating critical information during a procedure, and could impact its outcomes,” said Dr. Angelique Paulk of MGH, who is a co-author and co-inventor of the technology.
In addition, the electrodes currently used to monitor brain activity during surgery do not produce detailed fine grained data. So surgeons need to keep a buffer zone, known as resection margin, of 5 to 7 millimeters (about ¼ of an inch) around the area they are removing inside the brain. This means that they might leave some harmful tissue in. The new device provides a level of detail that would shrink this buffer zone to less than a millimeter.
“We invented the brain microdisplay to display with precision critical cortical boundaries and to guide neurosurgery in a cost-effective device that simplifies and reduces the time of brain mapping procedures,” said Shadi Dayeh, the paper’s corresponding author and a professor in the Department of Electrical and Computer Engineering at the UC San Diego Jacobs School of Engineering.
Researchers installed the LEDs on top of another innovation from the Dayeh lab, the platinum nanorod electrode grid (PtNRGrid). Using the PtNRGrids since 2019, Dayeh’s team pioneered human brain and spinal cord mapping with thousands of channels to monitor brain neural activity. They reported early safety and effectiveness results in a series of articles in Science Translational Medicine in 2022 in tens of human subjects.