PROCEPT BioRobotics
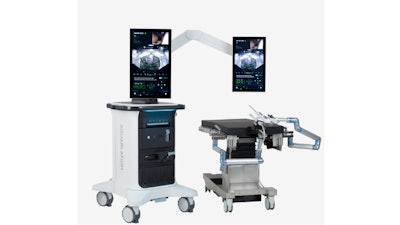
PROCEPT BioRobotics announced FDA 510(k) clearance of its platform, the HYDROS Robotic System, which features FirstAssist AI treatment planning, advanced image guidance, robotic resection, and a streamlined workflow.
The HYDROS Robotic System represents the next evolution in the delivery of Aquablation therapy, combining technology with user-friendly features designed to deliver better clinical outcomes for patients and healthcare providers.
Key Features of the Fully Integrated HYDROS Robotic System Include:
- AI-Powered Treatment Planning: FirstAssist AI, built from a library of real-world Aquablation therapy procedures, uses advanced image recognition software to accurately identify critical anatomy on ultrasound and suggest an optimal treatment plan for each patient.
- Advanced Image Guidance: The system integrates next-generation ultrasound imaging, digital cystoscopy, and dual high-definition touchscreens providing enhanced visualization of the anatomy and simultaneous viewing of ultrasound and cystoscopy images.
- Robotic Resection: Using a heat-free waterjet, the robot executes the surgeon-defined treatment plan to resect obstructive tissue while protecting critical anatomy. This enables efficient and predictable waterjet execution, standardizing the operative experience across a wide range of prostate sizes and shapes.
- Streamlined Workflow: Designed to improve the surgeon and staff experience at every stage of the Aquablation therapy procedure. With a single-footprint, the integrated tower facilitates efficient operating room setup and turnover. The adjustable touchscreen improves surgeon ergonomics with midline placement, and the instinctive software interface simplifies procedural workflow.