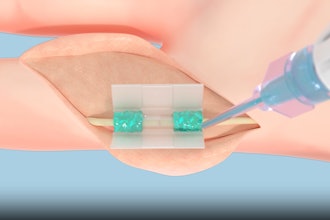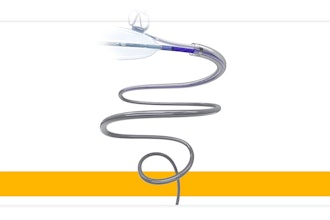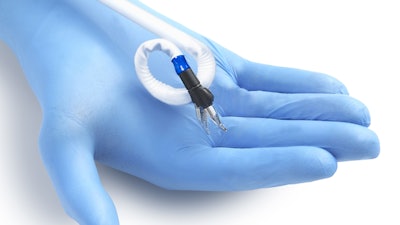
Momentis Surgical said the FDA has granted 510(k) clearance to its Anovo robotic surgical platform for use in single site, abdominal access ventral hernia repair. The new FDA clearance announced complements the system's existing approvals in natural orifice laparoscopic-assisted transvaginal benign gynecology surgical procedures.
The company said Anovo is the world's first single port robotics platform approved by the FDA to perform ventral hernia procedures. Performing procedures through a single port with multiple flexible instruments has the potential to result in less invasive and less traumatic outcomes for the tissue at the point of entry, enabled by the small portable robotic system.
The Anovo system is one of the first FDA-authorized surgical robotic platforms that feature miniature humanoid-shaped robotic arms that provide multi-planar flexibility and 360 degrees of articulation. The biomimetic instruments are designed to replicate the motions and capabilities of a surgeon's arms, with shoulder, elbow and wrist joints. Multiple instruments can be introduced to the body through a single portal and the 360-degree articulation offers obstacle avoidance as well as optimal access and working angles.