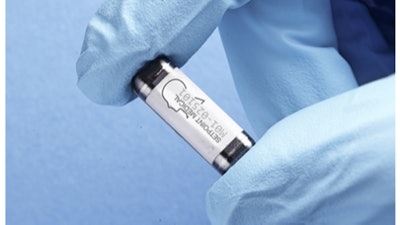
SetPoint Medical said that the FDA has approved its SetPoint System, a neuroimmune modulation device for the treatment of adults living with moderate-to-severe rheumatoid arthritis (RA) who are not adequately managed by—or cannot tolerate—existing advanced RA therapies, such as biological and targeted synthetic disease-modifying anti-rheumatic drugs (DMARDs).
“The approval of the SetPoint System, the first-in-class neuroimmune modulation platform, represents a transformative milestone in the management of autoimmune diseases,” said Murthy V. Simhambhatla, Ph.D., CEO of SetPoint Medical. “We are committed to improving the health of people living with RA, and look forward to working with providers and payers to make our innovative therapy accessible to their patients. We plan to introduce the SetPoint System in targeted U.S. cities this year, followed by expansion across the country starting in early 2026.”
The SetPoint System is an implantable, integrated neurostimulation device designed to deliver electrical stimulation to the vagus nerve, once daily, to activate the body’s innate anti-inflammatory and immune-restorative pathways.
FDA approval is supported by the results of the 242-patient randomized, double-blind, sham-controlled, RESET-RA study, that demonstrated the SetPoint System's safety and efficacy in patients with moderately to severely active RA who had an incomplete response or intolerance to one or more biologic or targeted synthetic DMARDS.
The device placement procedure and stimulation therapy were well-tolerated, with a low rate of related serious adverse events (1.7%), and no observations of malignancies, major cardiac events, or serious infections related to the SetPoint Therapy.
The SetPoint System received Breakthrough Device Designation from the FDA, a program designed for technologies that offer potentially more effective treatment or diagnosis for debilitating diseases. The company is also planning to evaluate its platform for treatment of additional autoimmune indications, including multiple sclerosis and Crohn’s disease.