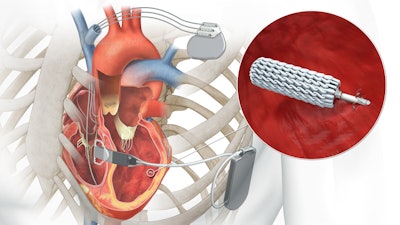
EBR Systems has received FDA approval of the WiSE System, designed to deliver leadless left ventricular endocardial pacing (LVEP) that aligns with the heart's natural conduction pathway. The endocardial approach represents a more physiological method of resynchronization and allows electrophysiologists to treat patients that are not able to receive lead-based devices.
"Recent years have brought significant advancements in leadless pacing for the right heart, but CRT patients were limited to traditional options—until now," said principal investigator Niraj Varma, MD, PhD, FRCP, Professor of Medicine at Cleveland Clinic. "Now, the WiSE System brings a leadless solution to left ventricular pacing, eliminating the biggest limitation of conventional CRT: the lead. This is a game-changer for patients who were previously untreatable due to anatomy or lead failures."
The WiSE System was designed for:
- Patients with challenging anatomy where the LV lead could not be implanted
- Patients with acute or chronic LV lead failure
- Patients with high procedural risk for LV lead placement
- Patients with leadless pacemakers who need CRT, yet are often poor candidates for conventional upgrades
The WiSE System syncs with existing pacing devices—pacemakers, ICDs, or CRTs—using a subcutaneous ultrasound Transmitter to power an ultra-compact Electrode implanted in the LV.






















