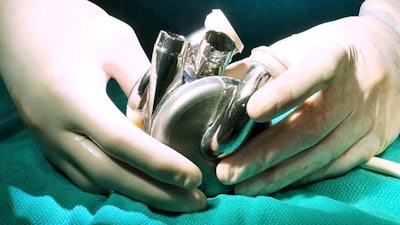
BiVACOR, a clinical-stage medical device company developing the world’s first titanium Total Artificial Heart (TAH), announced that it has received Breakthrough Device Designation from the FDA.
The designation supports the BiVACOR TAH as a bridge to transplant (BTT) for adults with severe biventricular or univentricular heart failure where current treatments, including LVADs, are not viable. The FDA’s Breakthrough Device program is reserved for technologies that may significantly improve outcomes for patients with life-threatening or irreversibly debilitating conditions. It offers priority regulatory interaction and accelerated pathways to approval.
The milestone follows the first phase of BiVACOR’s FDA Early Feasibility Study, where five patients in the U.S. received the TAH between July and November 2024. Based on positive safety and performance data, the FDA approved the expansion of the trial to include 15 additional patients starting later this year.
BiVACOR’s device represents a new category in artificial heart technology. Compact enough to fit most men and women, the TAH uses magnetic levitation, similar to maglev trains, to suspend a single dual-sided rotor. This rotor simultaneously powers the right and left circulatory systems, mimicking the natural heartbeat without valves or mechanical wear points. Its simplified design allows for pulsatile flow, large blood gaps to reduce trauma, and long-term durability.
The BiVACOR TAH is currently investigational and not approved for commercial use.






















