HistoSonics today announced its Edison Histotripsy System for the non-invasive destruction of liver tumors has been granted controlled early limited market access in Great Britain under a Unmet Clinical Need Authorization (UCNA). This designation, available through the UK’s Innovative Devices Access Pathway (IDAP) program, allows controlled early access to histotripsy for certain patients with liver tumors, marking a significant milestone in expanding treatment options for one of the UK’s most urgent unmet medical needs in oncology.
The IDAP program, launched by the UK Government to help fast-track transformative medical technologies into the healthcare system, selected the Edison System as one of just eight available application spots in 2024, based on histotripsy’s potential to meaningfully address the UK’s top current and future healthcare needs. Following a rigorous evaluation process, the UK’s medical device regulatory body (the Medicines and Healthcare products Regulatory Agency (MHRA) determined that our histotripsy devices could be made available through an UCNA, highlighting the important public health value this device may provide for patients with primary or metastatic liver tumors.
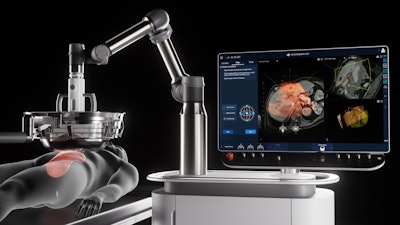
The Edison System is the first and only platform to use histotripsy, a non-thermal, non-invasive focused ultrasound technology, to mechanically liquefy tumors without surgery, radiation, or systemic therapies. The device received U.S. Food and Drug Administration (FDA) De Novo clearance in October 2023. The HistoSonics platform uniquely enables physicians to plan, target, treat, and monitor tumor destruction with continuous real-time visualization and control, offering unprecedented control and precision unmatched by any existing modality.
“Securing GB controlled access through the IDAP pilot program is a tremendous milestone for HistoSonics and signals clear recognition of our technology’s potential to transform healthcare,” said Mike Blue, President and CEO, HistoSonics. “We are honored to work alongside the NHS and UK regulatory and access partners to bring histotripsy to patients across the UK, many of whom have limited options. This progress builds on our strong clinical track record in the US, and our growing base of clinical evidence globally.”
Following FDA De Novo clearance in October 2023, the Edison System has been adopted at major academic centers and hospitals across the US, including integrated health systems and community hospitals who are also eager to offer this breakthrough, non-invasive treatment to patients. Additional clinical trials are ongoing to expand the use of histotripsy to additional indications, including the non-invasive treatment of kidney tumors and pancreatic tumors in advance of planned regulatory submissions for both indications.
The company believes strong clinical relationships within the UK, developed through multiple pioneering clinical trials conducted over previous years in both the liver and kidney, will help accelerate the adoption of histotripsy in major UK health centers.
As part of the UCNA authorization, HistoSonics will work with the MHRA to evaluate patient outcomes and regularly collect and analyze real-world data on device performance and safety as part of a post-market surveillance program. HistoSonics is concurrently pursuing European regulatory approval via the European Conformity process (CE marking), which will enable broader commercial availability across the UK and Europe.