BD announces the first pharma-sponsored combination product clinical trial using the BD Libertas Wearable Injector for subcutaneous delivery of complex biologics.
The pharma-sponsored combination product clinical trial represents a significant advancement in accelerating innovation in drug-device combination products that provide greater flexibility for patients, including potential conversion from infused medications that require patients to travel to a hospital or clinic to more convenient patient care in various settings, including self-injection at home.
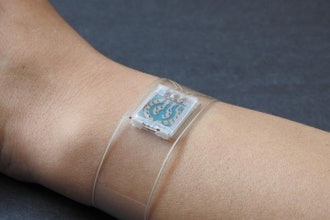
The BD Libertas Wearable Injector is a prefilled, ready-to-use drug delivery system designed to enable delivery of complex biologics via subcutaneous injection. The biologics market is expected to grow to more than $670 billion by 2030 and for pharmaceutical companies developing these complex drugs, the BD Libertas Wearable Injector offers a customizable, patient-centric solution. The BD Libertas Wearable Injector:
- Supports delivery of high-viscosity biologics (up to 50 centipoise), enabling a wide range of subcutaneous therapies
- Is offered in 2 to 5 mL and 5 to 10 mL configurations providing flexibility for diverse therapeutic requirements
- Features a fully mechanical, patient ready-to-use design with a "peel, stick and click" mechanism, requiring no end-user filling or assembly