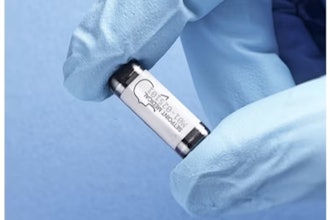
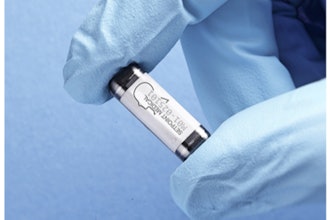
Samsara Vision said it completed the first U.S. surgeries of its SING IMT (Smaller-Incision New-Generation Implantable Miniature Telescope), as part of an FDA study to evaluate improvements in visual acuity and safety of the device in people living with late-stage age-related macular degeneration (AMD).
David RP Almeida, MD, MBA, PhD, from Erie Retinal Surgery (PA), and Marc H. Levy, MD, from the Sarasota Retina Institute (FL), performed the first procedures using the SING IMT in late June.
The trial will recruit 100 adults aged 65 and older living with stable (non-active neovascularization), bilateral central scotomas (blind spots) due to late-stage AMD and fovea-involving geographic atrophy or disciform scar to receive a SING IMT in one eye. In addition, candidates cannot have had previous cataract surgery in the study eye and must agree to post-operative comprehensive visual rehabilitation and training.
“It’s highly encouraging that our first surgeries went smoothly. We look forward to working with the eye health community across the country during the CONCERTO study. Our goal is to bring our new technology to people blinded by late-stage AMD across the United States,” said Thomas Ruggia, Chief Executive Officer at Samsara Vision. “We intend to work closely with the FDA to determine a timely pathway to bring the SING IMT™ to market in the United States.”
The SING IMT is a Galilean-style telescope implant designed to improve visual acuity and quality of life for patients with late-stage AMD. It is implanted during typical, out-patient cataract surgery with a corneal incision range between 6.5 mm to 7.5 mm. Images seen in “straight-ahead” vision are magnified 2.7x and projected onto healthy, undamaged areas of the macula in the back of the eye, reducing the apparent impact of the AMD “blind spot” on central vision. The SING IMT is approved for late-stage AMD patients who are 55 years of age or older in CE Referenced Countries, but it is not currently FDA approved in the United States.
Samsare warned that SING IMT is not a cure for late-stage AMD and that it will not return vision to the level a patient had before AMD, nor will it completely make up for vision loss.