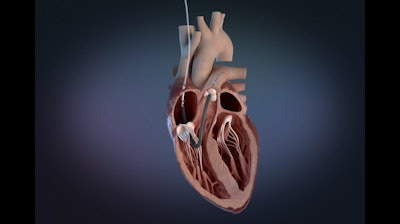
Abiomed said that Impella RP Flex with SmartAssist has received FDA pre-market approval (PMA), as safe and effective to treat acute right heart failure for up to 14 days. Impella RP Flex is implanted via the internal jugular (IJ) vein, which enables patient mobility, and has dual-sensor technology designed to optimize patient management.
The Impella RP platform includes what the company called the world’s smallest percutaneous right heart mechanical circulatory support (MCS) technologies designed to help patients achieve native heart recovery. They do not require extracorporeal blood circulation and remain the only MCS technologies with FDA PMA indications for the treatment of right heart failure.
Key clinical benefits of Impella RP Flex include:
- Single venous access via the internal jugular (IJ) vein and 11 French (Fr) indwelling catheter, facilitating patient mobility
- Flexible cannula advanced over an extra-support guidewire, enabling ease of insertion and pump delivery
- SmartAssist dual-sensor technology with Impella Connect, providing advanced metrics to help with pump management and weaning
- Heparin-free purge, simplifying patient anticoagulant management with the use of sodium bicarbonate where heparin is of concern due to heparin intolerance or bleeding






















