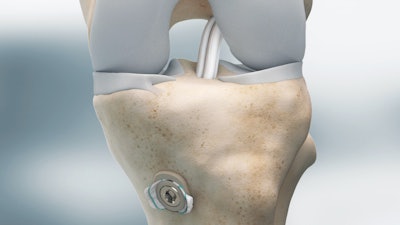
ABANZA Tecnomed, a designer and manufacturer of high-quality innovative Sports Medicine products, is announcing the FDA 510K Clearance of WasherCapTM, a soft tissue fixation system for ACL reconstruction.
The WasherCap is the first soft-tissue graft fixation device designed to allow surgeons to perform a fixation non-dependent on the bone quality of the patient and with precision and full control. The WasherCap has been designed to provide reliable fixation of the soft-tissue graft in ACL reconstructions. Due to the design, a complete entrapment of the soft-tissue is achieved inside the device, without depending on the bone quality of the patient and respecting the biomechanics of the knee.
Biomechanical tests have demonstrated that ACL reconstructions using the WasherCap device provide a higher fixation strength on the tibial side compared to standard tibial fixation devices such as interference screws. The WasherCap can be used in primary surgeries, nevertheless it is remarkably for ACL reconstruction revisions and for female patients where the bone quality may be compromised.
ACL reconstructions are among the most common sports medicine procedures performed in the United States, with around 300.000 injuries each year. Increasing participation in the recent years in youth sports, female sports and sport specialization are all likely to contribute to the increase of ACL injuries in young adults and female patients. It is estimated that the WasherCap could become the best fixation system for 40% of the reconstructions of anterior cruciate ligament in the USA.
"The FDA clearance of WasherCap is exciting because it offers surgeons a highly reliable solution for soft-tissue graft fixation, especially valuable for young, adults and female patients as well as revision procedures. WasherCap provides a stronger and more rigid tibial fixation, which will support a better ACL surgery outcome", Juan Abascal, Chief Executive Officer at ABANZA.






















