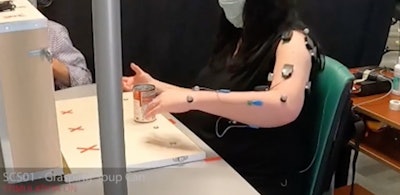
A neurotechnology that stimulates the spinal cord instantly improves arm and hand mobility, enabling people affected by moderate to severe stroke to conduct their normal daily activities more easily, report researchers from the University of Pittsburgh and Carnegie Mellon University today in Nature Medicine.
A pair of thin metal electrodes resembling strands of spaghetti implanted along the neck engage intact neural circuits, allowing stroke patients to fully open and close their fist, lift their arm above their head or use a fork and knife to cut a piece of steak for the first time in years.
“We discovered that electrical stimulation of specific spinal cord regions enables patients to move their arm in ways that they are not able to do without the stimulation. Perhaps even more interesting, we found that after a few weeks of use, some of these improvements endure when the stimulation is switched off, indicating exciting avenues for the future of stroke therapies,” said corresponding and co-senior author Marco Capogrosso, Ph.D., assistant professor of neurological surgery at Pitt. “Thanks to years of preclinical research building up to this point, we have developed a practical, easy-to-use stimulation protocol adapting existing FDA-approved clinical technologies that could be easily translated to the hospital and quickly moved from the lab to the clinic.”
When it comes to strokes, doctors predict a grim future: Globally, every fourth adult over the age of 25 will suffer a stroke in their lifetime, and 75% of those people will have lasting deficits in motor control of their arm and hand, severely limiting their physical autonomy.
Currently, no treatments are effective for treating paralysis in the so-called chronic stage of stroke, which begins approximately six months after the stroke incident. The new technology, researchers say, has the potential to offer hope for people living with impairments that would otherwise be considered permanent.
“Creating effective neurorehabilitation solutions for people affected by movement impairment after stroke is becoming ever more urgent,” said senior co-author Elvira Pirondini, Ph.D., assistant professor of physical medicine and rehabilitation at Pitt. “Even mild deficits resulting from a stroke can isolate people from social and professional lives and become very debilitating, with motor impairments in the arm and hand being especially taxing and impeding simple daily activities, such as writing, eating and getting dressed.”
Spinal cord stimulation technology uses a set of electrodes placed on the surface of the spinal cord to deliver pulses of electricity that activate nerve cells inside the spinal cord. This technology is already being used to treat high-grade, persistent pain. Additionally, multiple research groups around the world have shown that spinal cord stimulation can be used to restore movement to the legs after spinal cord injury.
But the unique dexterity of the human hand, combined with the wide range of motion of the arm at the shoulder and the complexity of the neural signals controlling the arm and hand, add a significantly higher set of challenges.
Following years of extensive preclinical studies involving computer modeling and animal testing in macaque monkeys with partial arm paralysis, researchers were cleared to test this optimized therapy in humans.
“The sensory nerves from the arm and hand send signals to motor neurons in the spinal cord that control the muscles of the limb,” said co-senior author Douglas Weber, Ph.D., professor of mechanical engineering at the Neuroscience Institute at Carnegie Mellon University. “By stimulating these sensory nerves, we can amplify the activity of muscles that have been weakened by stroke. Importantly, the patient retains full control of their movements: The stimulation is assistive and strengthens muscle activation only when patients are trying to move.”
In a series of tests adapted to individual patients, stimulation enabled participants to perform tasks of different complexity, from moving a hollow metal cylinder to grasping common household objects, such as a can of soup, and opening a lock. Clinical assessments showed that stimulation targeting cervical nerve roots immediately improves strength, range of movement and function of the arm and hand.
Unexpectedly, the effects of stimulation seem to be longer-lasting than scientists originally thought and persisted even after the device was removed, suggesting it could be used both as an assistive and a restorative method for upper limb recovery. Indeed, the immediate effects of the stimulation enable administration of intense physical training that, in turn, could lead to even stronger long-term improvements in the absence of the stimulation.
Moving forward, researchers continue to enroll additional trial participants to understand which stroke patients can benefit most from this therapy and how to optimize stimulation protocols for different severity levels.
Additionally, Pitt and CMU-founded startup Reach Neuro is working to translate the therapy into clinical use.
Marc Powell, Ph.D., of Reach Neuro Inc.; Nikhil Verma, B.S., of Carnegie Mellon University; and Erynn Sorensen, B.S., of Pitt are co-first authors. Additional authors of the study are Erick Carranza, B.S., Amy Boos, M.S., Daryl Fields, M.D., Ph.D., Souvik Roy, B.S., Scott Ensel, B.S., Jeffrey Balzer, Ph.D., Robert Friedlander, M.D., George Wittenberg, M.D., Ph.D., Lee Fisher, Ph.D., and Peter Gerszten, M.D., all of Pitt; Beatrice Barra, Ph.D., of New York University; Jeff Goldsmith, Ph.D., of Columbia University; and John Krakauer, Ph.D., of Johns Hopkins University.
Research reported in this press release was supported by the NIH BRAIN Initiative under Award number UG3NS123135. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Additional research support was provided by the Department of Neurological Surgery and the Department of Physical Medicine and Rehabilitation at Pitt, and the Department of Mechanical Engineering and the Neuroscience Institute at Carnegie Mellon University.






















