Medical implants can be lifesaving or significantly improve quality of life, but to our immune system they can appear as intruders.
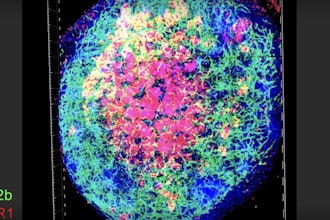
Rice University bioengineer Omid Veiseh and collaborators have found that lipid deposition on the surfaces of implants can play a mediating role between the body and implants, with some lipids acting as peacekeepers while others stir up conflict.“We learned that as immune cells crawl on an implanted biomaterial, they leave lipid vesicles that signal to the host immune system whether the biomaterials should be ignored or walled off from the body,” said Veiseh, a Rice assistant professor of bioengineering and Cancer Prevention and Research Institute of Texas scholar.
This knowledge could help scientists develop biomaterials or coatings for implants that deflect host immune system aggression, reducing malfunction rates for biomedical devices such as pacemakers, cerebrospinal fluid shunts, coronary stents, surgical mesh, drug delivery pumps, biosensors and more.
The study is published in Advanced Materials.
“A major problem in all biomedical implants is that the immune system attacks them,” said Christian Schreib, a Rice graduate student and lead author on the study. “Essentially, it encapsulates them in a fibrotic capsule that destroys their functionality and makes them not work anymore.”
“Our team was able to develop a chemical surface modification that preferentially recruits macrophages which leave behind a ‘do-not-attack’ lipid-vesicle signature allowing implants to exist in the body without being recognized as foreign,” Veiseh said.
Fibrosis, or scarring, is the accumulation of excess tissue at the site of an injury. The fibrotic response to implants has traditionally been associated with the deposition of proteins on the implanted surface.“In our research, we realized that, while proteins are important, fat molecules also play a significant role in the fibrotic process,” Schreib said. “We identified two lipid profiles, fatty acids and phospholipids. Fatty acids are more likely to provoke an immune response, while phospholipids are more likely to fly under the radar and not irk the immune system.
“Now that we understand this, we can use this knowledge to engineer materials for use in implants that are less likely to trigger an immune response. We could, say, engineer a material that pulls in phospholipids to it, so that when you implant the material, the phospholipids naturally deposit onto it and help it evade the immune system. We might also want to look at taking those fat molecules like the phospholipids and chemically functionalize them to the device surface before implantation.”
When an immune response is triggered in the body, immune cells are mobilized at the site of the injury or intrusion. The increased traffic of immune cells near the implant leads to a greater accumulation of fibrotic tissue.
“A thick layer of cells deposited on the implant is likely to stop it from working,” Schreib said. “But if you have a layer of lipids on the atomic scale, that’s not going to affect its functionality to the same extent.”Optimizing implant performance is most critical for patient groups who rely on them for the management of chronic and potentially life-threatening conditions such as hydrocephalus, a disorder that involves an excess buildup of cerebrospinal fluid (CSF) in the brain. For many patients, the only effective management strategy is the placement of a CSF shunt that diverts excess fluid to a different body cavity. Pediatric hydrocephalus patients face particularly high rates of implant failure, which can result in headaches, vomiting, loss of vision, brain injury and death if not addressed quickly.
“As a pediatric neurosurgeon, it's safe to say that shunt malfunctions are the bane of my existence,” said Dr. Brian Hanak, assistant professor of neurosurgery at Loma Linda University Children’s Hospital in California who is a co-author on the study. While CSF shunt malfunction can occur in any age group, malfunction rates are much higher in young children. “Most of us working in this field feel that is likely related to the fact that the brain’s innate immune system is particularly revved up in young children,” he said.
“In young children and babies, shunt malfunction rates are in the ballpark of 40%-50% at two years post-implantation. Frankly, I'm embarrassed to routinely implant the most failure-prone life-sustaining device in modern medicine. If you developed a pacemaker with a 40% to 50% failure rate at two years, it would never get approval from the U.S. Food and Drug Administration, because that's appalling. But that's unfortunately the industry standard for CSF shunts.”
Hanak said many brain implants could benefit from a reduced innate immune response.“One in particular that always comes to mind for me is brain-computer interface technology,” he said. “It's been about 20 years now that we've had proof-of-concept that you can implant a microelectrode array in someone's brain and have them use that array to control a robotic arm.“You might ask, if that’s the case, then why is this technology not something that every paralyzed person can use to improve their independence and quality of life? The reason is that the immune response mounted to those implanted electrode arrays makes them unable to record neural activity beyond two to three years in vivo. At the moment, with our current state of technology, it's not really a viable solution, certainly not a long-term solution for paralyzed patients.”