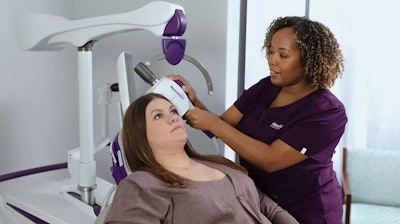
Neuronetics, a medical technology company focused on designing, developing, and marketing products for patients who suffer from neurohealth disorders, announced the FDA clearance of NeuroSite Coil Placement Accessory, a tool that simplifies measurement and coil positioning during NeuroStar transcranial magnetic stimulation (TMS) treatments. This proprietary accessory, designed with versatility and provider convenience in mind, seamlessly integrates with both legacy and new NeuroStar systems and further enhances efficiency and patient experience.
“The NeuroSite FDA clearance is the latest of many enhancements designed to improve the patient experience, exemplifying our ongoing commitment to continually advance medical technology for mental health,” stated Cory Anderson, SVP of R&D and Clinical. “We are thrilled to introduce yet another innovative accessory to our suite of products that deliver a fast, easy, reliable treatment that both patients and providers trust.”
By leveraging the patient's unique anatomical features, NeuroSite Coil Placement Accessory ensures precise and reproducible coil placement. This streamlines the NeuroStar TMS process with significantly fewer patient setup steps, thereby improving efficiency for providers’ offices and improving the patient experience while maintaining the accuracy established by the current NeuroStar Head Support System (HSS).
“As a NeuroStar TMS provider with multiple systems, I appreciate how user-friendly NeuroSite is, along with the flexibility it brings to patient positioning across all generations of NeuroStar devices,” said Dr. Kenneth Pages, Medical Director at TMS of South Tampa. “This latest tool reinforces my confidence in providing consistent results and truly exceptional care to my patients.”
NeuroSite™ will be available to all NeuroStar providers beginning in Q1 of 2024.