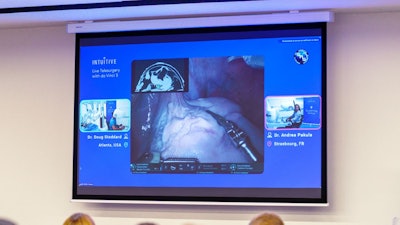
Intuitive, a company specializing in robotic-assisted surgery, has demonstrated its telesurgery capabilities by remotely connecting two surgeons to perform a transatlantic demonstration at the Society of Robotic Surgery (SRS) conference in Strasbourg, France.
The event involved Doug Stoddard, MD, located in Peachtree Corners, Georgia, and Andrea Pakula, MD, located remotely in Strasbourg, France. Together, using a dual console da Vinci 5 system, they performed a telesurgery procedure on an advanced tissue model developed by Intuitive to replicate the behavior of live tissue.
During the demonstration, Stoddard, whose console was alongside the da Vinci 5 patient cart and tissue model, was able to pass control of surgical instruments back and forth remotely to Pakula, located at the remote surgeon console. This included Force Feedback, enabling both surgeons to feel the forces exerted on the advanced tissue model by the instruments, despite being more than 4,000 miles apart.
Telesurgery — the ability of a surgeon to perform an operation remotely from the patient via a robotic surgical system — became feasible in 2001 when surgeons performed the first transatlantic procedure, dubbed Operation Lindbergh, between the U.S. and Strasbourg, France.
“While not a new idea for Intuitive, telesurgery requires a high performing network infrastructure and a robotic system designed for remote collaboration to be successful and sustainable. Our focus is not on being first but on being rigorous in building the infrastructure to support safety, reliability, and consistent use,” said Brian Miller, PhD, Intuitive’s executive vice president and chief digital officer, who was an engineer supporting Operation Lindbergh.
The telesurgery software on the da Vinci 5 system that was shown during this demonstration was used for demonstration purposes only. The technology is still in development, is not 510(k) cleared or CE marked, and the safety and effectiveness of the product has not been established. The technology is not currently for sale and cannot be made available nor put into service in the U.S. or in the European Union.