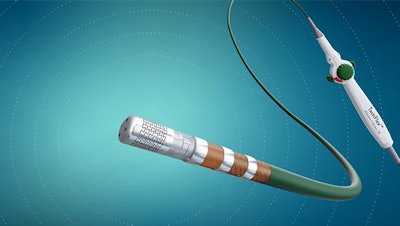
Abbott today announced that the U.S. Food and Drug Administration (FDA) has approved the company's TactiFlex Ablation Catheter, Sensor Enabled, the world's first ablation catheter with a flexible tip and contact force technology.
Used to perform an ablation procedure to treat atrial fibrillation (AFib), the most common abnormal heart rhythm, the TactiFlex catheter can result in reduced procedure times and better safety when compared to the company's previous generation catheters.
More than 37 million people worldwide live with AFib and numbers are predicted to more than double by 2050. An additional five million cases are diagnosed every year, indicating a growing health challenge that demands innovative solutions for patients and their physicians.
"For those suffering from AFib, daily life can be challenging as people often feel dizziness, chest pain and heart palpitations. AFib can lead to stroke if left untreated, making it critical for physicians to treat the issue as early as possible," said Larry A. Chinitz, M.D., director of the Heart Rhythm Center and co-director of NYU Langone Heart in New York City. "We are entering the next chapter of AFib ablation with new tools such as Abbott's TactiFlex that, when used with mapping systems to accurately identify the source of an arrhythmia, can safely and efficiently treat the problem in ways we never thought possible a decade ago."
The TactiFlex catheter is designed to be used with Abbott's EnSite X EP System, an industry-leading heart mapping system, which allows physicians to view and precisely identify areas in the heart that require ablation.
Unlike other catheters on the market, the TactiFlex catheter uses a tip design with a laser-cut pattern that flexes when in contact with the heart wall. This helps direct fluid to the treated tissue and allows for more accurate positioning of the catheter – providing up to two-times higher stability in a beating heart – for consistent therapy delivery.
"Abbott is leading the way in helping doctors manage common arrhythmias with the most holistic portfolio for this condition in the world," said Christopher Piorkowski, M.D., chief medical officer of Abbott's electrophysiology business. "The EnSite X EP System is unmatched in determining the exact location where ablation is required. Coupled with the TactiFlex catheter, patients can now feel even more confident that their procedure will deliver safe and effective results."
The Abbott TactiFlex catheter generated strong clinical outcomes in the TactiFlex AF IDE study. The study showed the catheter created fast, safe lesions to treat AFib with over 99% acute procedural success.
The TactiFlex catheter is also approved for use in Europe, Japan, Africa and Australia.