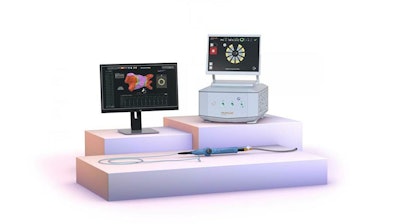
Biosense Webster today announced European CE mark approval of the VARIPULSE Platform for the treatment of symptomatic drug refractory recurrent paroxysmal atrial fibrillation (AF) using pulsed field ablation (PFA). The VARIPULSE Platform is comprised of the VARIPULSE Catheter, a variable-loop multielectrode catheter; the TRUPULSE Generator, a multichannel PFA generator; and CARTO 3 System, the world’s leading 3D cardiac mapping system. The VARIPULSE Platform is a CARTO-integrated PFA system, enabling a reproducible workflow with real-time visualization and feedback mechanisms.
The safety and efficacy of the VARIPULSE Platform was investigated in the inspIRE trial, which included 186 patients in Canada and Europe. Updated one-year follow-up data was presented this month at the AF Symposium in Boston, demonstrating that among participants receiving optimal PFA applications, 80% achieved freedom from recurrence with zero primary adverse events. Furthermore, the primary effectiveness endpoint (PEE) of acute pulmonary vein isolation and 12-month freedom from atrial arrhythmia recurrence (AF, Atrial Tachycardia, or Atrial Flutter) was 75.6%. The study reported a low fluoroscopy time of 7.8 minutes, partly attributed to the integration of the VARIPULSE Catheter to the CARTO 3 System and a good safety profile with no (0.0%) primary adverse events reported.
Catheter ablation is a minimally invasive procedure performed by an electrophysiologist to treat heart rhythm disorders, including AF, by interrupting irregular electrical pathways in the heart by delivering either heat (radiofrequency ablation) or cold (cryoablation). PFA represents a new approach to treating AF, utilizing a controlled electric field to selectively ablate cardiac tissue that causes the irregular heartbeat through a process called irreversible electroporation (IRE). Because the pulsed field energy is minimally thermal, IRE offers the potential to reduce the risk of damage to surrounding tissues including esophageal, pulmonary vein, and phrenic nerve injury.
“At Biosense Webster, we continually seek to push the boundaries of science and technology innovation in cardiac ablation. CE mark approval of the VARIPULSE Platform is testament to this, now offering healthcare professionals the potential to improve outcomes for people living with atrial fibrillation while setting a new standard in cardiac electrophysiological mapping,” said Jasmina Brooks, President, Biosense Webster. “We believe pulsed field ablation has the potential to offer safer, more consistent and efficient workflows, and the VARIPULSE Platform uniquely offers physicians a simple and reproducible PFA workflow with 3D visualization, in real-time.”






















