Boston Scientific has received FDA approval to expand the indication for current-generation INGEVITY+ Pacing Leads – thin wires placed inside the heart and connected to an implantable device – to include conduction system pacing (CSP) and sensing of the left bundle branch area (LBBA) when connected to a single- or dual-chamber pacemaker.
Pacing of the LBBA is an alternative to traditional right ventricular pacing for the treatment of symptomatic bradycardia, a condition in which the heart beats too slowly. Through this pacing approach, which uses the heart's natural electrical system, a lead is placed in the LBBA of the heart's conduction system. This technique may promote greater ventricular synchrony and reduce the long-term risk of heart failure associated with traditional right ventricular pacing.
Clinical evidence submitted to the FDA to support the expanded indication included data from approximately 400 patients from the INSIGHT-LBBA study – an analysis of INGEVITY+ pacing leads that were previously implanted in the LBBA for anti-bradycardia pacing – and supplemented with bench testing and LATITUDE™ Programming System data.
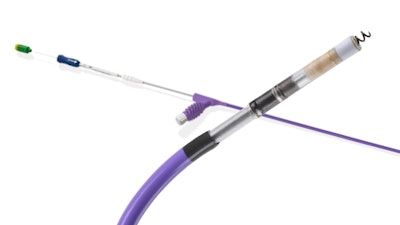
The INGEVITY+ Pacing Lead is driven by a stylet during lead placement, which supports positioning the device into a desired location within the heart and allows for both continuous pacing and impedance monitoring – key features that can aid appropriate placement and fixation. The expanded indication follows the launch of the Boston Scientific CSP portfolio – inclusive of the OneLINK™ Splitter Cable, INGEVITY+ Helix Locking Tool and site-selective pacing delivery catheters – which is designed to support the safe and effective placement of the INGEVITY+ Pacing Lead in the LBBA. The INGEVITY+ lead received FDA approval in 2019 for use with pacemakers and defibrillators.