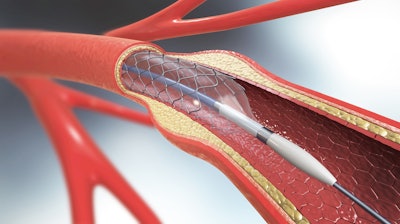
Medtronic announced it received U.S. Food and Drug Administration (FDA) approval for the Onyx Frontier drug-eluting stent (DES) with an enhanced delivery system designed to improve deliverability and increase acute performance in even the most challenging of cases.
The Onyx Frontier DES is used for the treatment of patients with coronary artery disease (CAD), which is caused by plaque buildup on the inside of the coronary arteries. These plaque deposits can narrow or clog the inside of the arteries, which decreases the supply of blood and oxygen to the heart. CAD is the leading cause of death for both men and women in the United States. To help to restore blood flow, a physician may use a stent (a flexible metal scaffolding) that is delivered during a minimally invasive procedure to prop open the artery. A drug-eluting stent is the most common type of stent used to treat a blockage of the heart arteries.
"The new Onyx Frontier DES, with its enhanced deliverability, will continue to help interventional cardiologists treat complex coronary cases and larger ranges of vessel sizes more efficiently," said Azeem Latib, M.D., section head of interventional cardiology and medical director of structural heart interventions at Montefiore Medical Center in New York City. "Delivering safe and effective outcomes to our patients is our number one priority. It's important that physicians have access to tools like the Onyx Frontier DES that can allow them to efficiently achieve those outcomes."
Meaningful design changes, including increased catheter flexibility, an innovative dual-layer balloon technology and a lower crossing profile led to a 16% improvement in deliverability with Onyx Frontier vs. the previous generation Resolute Onyx DES. In addition to the delivery system enhancements, Onyx Frontier offers a broad size matrix to treat more patients and is the only 2.0 mm DES available in the United States (similar to Resolute Onyx). Further, Onyx Frontier continues to provide 4.50-5.00 mm sizes that can be expanded to 6.00 mm - specifically designed to support extra-large vessels. Onyx Frontier shares the same clinical indications as Resolute Onyx, including the most recent approval for patients that are at high risk of bleeding who may benefit from a dual antiplatelet therapy (DAPT) duration as short as one month.
The Onyx Frontier DES is now approved in the United States and is pending CE (Conformité Européene) Mark.


















