It's not just for warding off werewolves. Silver's antimicrobial properties have seen various applications throughout human history for at least six millennia and are still present in medicine today.
Researchers of American Chemical Society Central Science (ACS) are coating internal medical implants with a proprietary silver mixture and seeing overwhelming success in preventing infection.
Numbered 47 on the Periodic Table of Elements with the symbol Ag, silver's discovery is unclear. It first appears historically in uncovered smelting setups dating to 4000 BC and is thought to have accrued value as currency very quickly next to gold and copper. This is perhaps due to silver being the most reflective ore, despite its otherwise lack of structural practicality as a metal.
Silver's antibacterial properties were employed to preserve food and water by ancient Roman, Phoenician, Egyptian, and Greek Societies. Macedonians and Hippocrates used silver compounds in healing wounds and warding off infection, believing compounds with Argentums, the Latin word for silver, could cure ulcers. Even American pioneers were known to keep silver coins in milk jugs to prevent spoiling.
Challenges Facing Researchers
Medical implants such as pacemakers, tubes for drainage or administration of medicine, stints, screws, etc., all face the potential for bacteria collection. This consistent collection of bacteria is called a biofilm, "a thin, slimy film of bacteria that adheres to a surface" and is difficult to prevent in indwelling medical devices.
Silver is used in dentistry, as a coating for external medical devices, in antibiotic creams, and as an ingestible supplement, but it has not been used effectively in medical implants due to its potential toxicity over long periods of exposure. Solving this problem was the main part of ACS researchers' goal, creating a silver compound that would effectively prevent infection while not poisoning patients.
Chemists also needed to make a silver compound that would consistently kill bacteria for extended periods of time, as silver is not an active antimicrobial for an indefinite period. This balance of team objectives is accurately described in the official ACS research study abstract published in April.
"The long-term prevention of biofilm formation on the surface of indwelling medical devices remains a challenge. Silver has been reutilized in recent years for combating biofilm formation due to its indisputable bactericidal potency; however, the toxicity, low stability, and short-term activity of the current silver coatings have limited their use," says ACS.
Silver toxicity and sustainable antimicrobial activity are obstacles best understood after learning how silver kills bacteria. Antimicrobial silver exists with a positive molecular charge that consistently releases ions in search of negative ions to balance its chemical structure.
Negative ions present in undesirable bacteria are ripped out, killing them. 2016 research found that bacteria soak up silver ions as they die, posing further sterilization to future bacteria.
The release of silver ions by positively charged silver cannot happen continuously forever. Antimicrobial silver must release ions for the time it needs to repel bacteria.
To create the ideal silver compound for use in medical implants, the team needed to make a silver mixture that would consistently release ions at a slower pace. This, in turn, would ensure the silver is less toxic to an individual host over long periods.
"SAFE" Coating, the Ideal Medical Silver
The ACS team developed and tested what they describe as "silver-based film-forming antibacterial engineered," abbreviated SAFE, to solve all of silver's inherent downfalls as an antimicrobial agent. This novel compound consistently released ions at a low rate did not cause toxicity in test subjects, and was easy to apply to various materials for potential use.
After working with several ingredients, chemists found success with a combination of silver nitrate, dopamine, and two hydrophilic polymers. Next, SAFE was tested in vitro and in vivo (both in live subjects and glass containers) over 28 days, alongside pure silver as a control group.
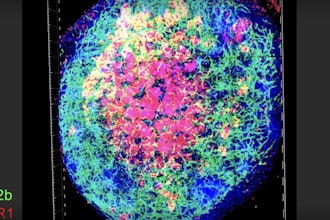
To test the efficacy of SAFE in living tissue, a titanium probe was coated with SAFE and placed beneath the skin of live rats. As demonstrated in the microscope imagery from the study, SAFE eradicated the bacteria and continued doing so for the entirety of the study.
This effect was evident compared to the control group.
Both SAFE and the control were tested against diverse bacteria, including E. coli, MRSA, and K. pneumoniae. SAFE proved highly effective, rendering bacteria nearly undetectable in its trial.
Future Steps
The ACS study concludes with, "The coating showed broad-spectrum antibiofilm activity, was able to prevent infection in a rat infection model and was found to be highly biocompatible in vitro and in vivo without silver toxicity. The current coating is anticipated to have broad application for diverse medical devices and implants to prevent implant-/device-associated infections."
SAFE represents a new frontier in medical instruments, specifically indwelling medical devices and implants. This study denotes ingenious chemistry, through which the ACS team skillfully harnessed the desirable qualities of a naturally occurring element while eliminating its harmful side effects.
Their resulting compound was shown to produce the desired result: eliminating bacteria biofilm consistently and with more longevity than pure silver and avoiding silver toxicity in rats. There is no plan for SAFE clinical human trials yet, but its potential is vast.