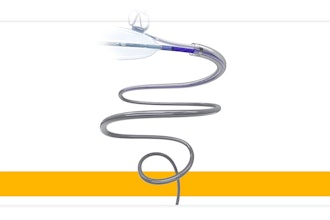
Serpex Medical announced today U.S. FDA 510(k) clearance of its Recon Steerable Sheath, a steerable endobronchial tool that offers articulation at the distal tip to enable physicians to access difficult-to-reach areas of the lung anatomy.
The company seeks to leverage the power of steerable instruments to enable greater precision and access to improve the diagnosis and treatment of lung cancer. The Recon Steerable Sheath is the first device cleared in Serpex Medical's product portfolio of steerable instruments.
"I've had the opportunity to trial this device. The ability to make adjustments and reposition, particularly at the target location, is phenomenal," says Michael Machuzak, MD, Interventional Pulmonologist at The Cleveland Clinic who participated in the preclinical testing. "This is an exciting and novel device that holds enormous potential for the future of bronchoscopy."
Lung cancer is the leading cause of cancer deaths worldwide. It has the lowest five-year survival rate (18%) when compared to other common cancers, in part because most cases are diagnosed late-stage.1 Approximately 70% of cancerous lung nodules are found in the outer third of the lung2 and diagnosing these cases early is challenging.
Serpex Medical is backed by Ajax Health, Zeus Health, Aperture Venture Partners and Western Technology Investment. The company's R&D pipeline includes a steerable needle among other devices leveraging its proprietary steerable device technology.
- "Cancer Stat Facts: Common Cancer Sites," National Cancer Institute, https://seer.cancer.gov/statfacts/html/common.html.
- "Cancer Stat Facts: Lung and Bronchus Cancer," National Cancer Institute, https://seer.cancer.gov/statfacts/html/lungb.html.