Exactech announced its subsidiary, BlueOrtho, has received 510(k) clearance from the Food and Drug Administration for ExactechGPS Ankle, what it's calling the world’s first surgical navigation system for total ankle arthroplasty (TAA).
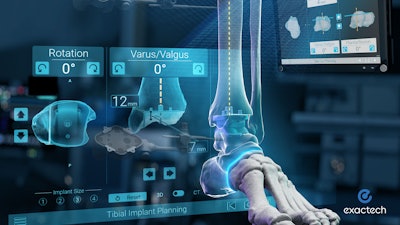
GPS Ankle is a technology, which connects the preoperative plan with real-time intraoperative instrument guidance and confirms that resections meet the surgical plan. The system uses proprietary active tracker technology and a compact touchscreen tablet in the sterile field to provide surgeons with dynamic intraoperative feedback throughout their cases.
GPS Ankle is compatible with Exactech’s flagship Vantage Total Ankle System, and will be available to hospitals and ASC centers without capital cost. Pre-clinical studies, based on bench testing, reported an accuracy of 2mm and 2 degrees relative to the CT-based surgical plan.1 This was further confirmed by two studies performed on sawbones, which were recently accepted by the Orthopaedic Research Society.
GPS Ankle is the latest product introduction of Exactech’s Active Intelligence dynamic ecosystem of enabling technologies and smart solutions that empowers surgeons with data-rich, comparatively low-cost solutions to help improve patient outcomes. The company’s GPS technology has been in use across the globe for more than a decade for shoulder and knee surgery.
GPS Ankle is only available in the U.S. and will enter pilot launch with limited availability starting in 2024. Contact your local Exactech rep for more information.