CurvaFix
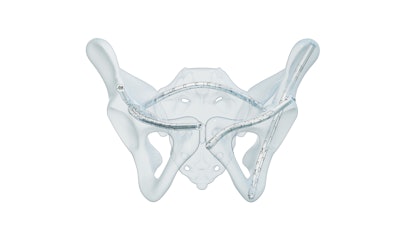
CurvaFix, a developer of medical devices to repair fractures in curved bones, today announced FDA 510(k) clearance for its next-generation CurvaFix Low Profile System, a percutaneous solution for fixation of pelvic fractures.
The CurvaFix Low Profile System is built to expand surgical options in challenging scenarios, including pathological bone, curved or narrow pelvic corridors, intersecting fixation pathways, and cases where indwelling or adjacent hardware is present. The new system offers:
- 65% smaller head geometry for a lower-profile construct
- Implants with increased compression capability
- Extended implant lengths up to 210mm, enabling connection of intraosseous fixation pathways with a single device
- Patented lock technology with improved visual and tactile confirmation






















