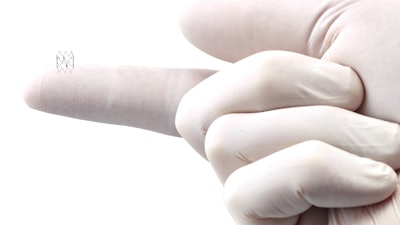
Royal Philips presented latest results from its Tack Optimized Balloon Angioplasty (TOBA) II below-the-knee (BTK) clinical trial, demonstrating that the Philips endovascular system – Tack (4F) – which is approved by the U.S. Food and Drug Administration (FDA), provides a sustained treatment effect and positive impact on quality of life for patients with critical limb ischemia (CLI), a severe stage of peripheral arterial disease (PAD), out to three years of clinical follow-up.
CLI occurs when an obstruction in an artery severely reduces blood flow, causing painful wounds, debilitating rest pain, recurring ulcers and life-threatening infection. If left untreated, 50% of patients with CLI will undergo an amputation or die within the first year.The TOBA II BTK trial studied the safety and efficacy of post-angioplasty dissection repair using the Philips Tack endovascular system in patients with CLI and infrapopliteal disease. The Tack endovascular system is a unique specialized implantable device to optimize the treatment of dissections in patients with CLI. Endpoints included rates of major adverse events, target lesion revascularization (TLR), target limb salvage (TLS) and quality of life (QoL) metrics.
“Based on 36-month follow-up in the TOBA II BTK trial, which is following 233 patients at 41 sites internationally, the Tack endovascular system offers a new standard in repairing below-the-knee arterial dissections,” said co-principal investigator George Adams, M.D., director of cardiovascular and peripheral vascular research at UNC Rex Hospital in Raleigh, N.C., and clinical associate professor of medicine at the University of North Carolina at Chapel Hill. “The device stands to significantly benefit patients with CLI whose treatment with balloon angioplasty often leads to this particular complication, which typically goes untreated and unaddressed, to the detriment of long-term clinical outcomes.”
The three-year analysis of TOBA II BTK results includes the following clinical insights on the Tack endovascular system.