UroMems, a company developing mechatronics technology to treat stress urinary incontinence (SUI), announced today that it has successfully completed the first-in-human implant of the UroActive System, a smart automated artificial urinary sphincter (AUS) investigational device to treat SUI. This initial clinical study is a key milestone in the development of UroActive.
"This is a historic milestone for UroMems, patients, physicians and the industry as this is a first-of-its-kind fully automated AUS implant designed to treat SUI in both men and women," said Hamid Lamraoui, UroMems chief executive officer and co-founder. "This is the next important step in delivering our novel technology to a large, underserved patient population with unmet needs not addressed by current options on the market."
The first male patient procedure was successfully completed at La Pitié-Salpêtrière University Hospital (AP-HP, Sorbonne University, Paris, France) by Professor Emmanuel Chartier-Kastler, principal investigator, with approval from the National Agency for the Safety of Medicines and Health Products (or ANSM, the French equivalent to the U.S. Food and Drug Administration).
"We believe this groundbreaking therapy will change the way SUI is treated worldwide, establishing a new standard of care," said Professor Pierre Mozer, UroMems chief medical officer and co-founder.
UroMems aims to restore the quality of life, dignity and self-esteem of millions of men and women worldwide suffering from untreated chronic conditions by the commitment to change the perception that these disorders are inevitable as one grows older and is simply something to endure with no real solution. UroMems is revolutionizing the treatment of SUI with smart active implants, using the latest technological advances in the field of embedded systems and micro-technologies for the development of its groundbreaking solutions.
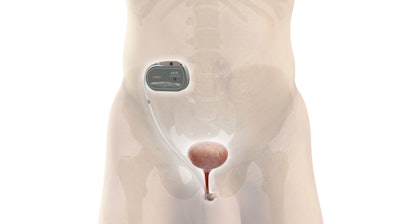
UroActive is the first smart active implant that treats SUI, powered by a MyoElectroMechanical System (MEMS). This innovative system is placed around the urethral duct and is automatically controlled based on the patient's activity, without the need for manual adjustments, intending to provide patients with ease of use and a better quality of life than current options.
"We see a high rate of today's AUS implants that are revised or removed after three years," said Professor Daniel Elliott, urologist at Mayo Clinic in Rochester, Minn., and clinical advisor to UroMems. "The potential of being able to personalize the therapy to the patient automatically, post-implantation is very appealing."
SUI, or involuntary urinary leakage, affects an estimated 40 million Americans and 90 million Europeans, and occurs when the pressure in the bladder exceeds that of the muscle (the sphincter) around the urethra, caused by activities involving high intra-abdominal pressure, like coughing, laughing and exercising. SUI significantly impacts quality of life, as it can be debilitating, and often leads to depression, low self-esteem and social stigma. While mild SUI is addressed by pelvic floor re-education and bulking agents, moderate and severe SUI historically have only had two options: mesh sling or artificial urinary sphincter.