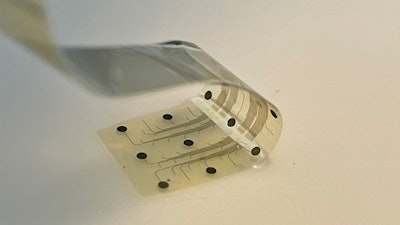
INBRAIN Neuroelectronics S.L., a health-tech company dedicated to developing the world’s first intelligent graphene-neural platform, today announced that its Intelligent Network Modulation System has been granted Breakthrough Device Designation (BDD) from the U.S. Food & Drug Administration (FDA) as an adjunctive therapy for treating Parkinson’s disease.
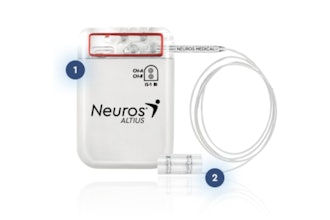
The INBRAIN system harnesses the power of graphene, a two-dimensional material made of a lattice of carbon atoms only one atom thick. The thinnest material known, yet stronger than steel, graphene’s unique combination of electrical and mechanical properties makes it ideal for neurotechnology innovation. INBRAIN’s neural platform technology enables ultra-high signal resolution at levels never seen before, and uses machine learning software that decodes therapy-specific biomarkers to deliver highly focused, adaptive neuroelectronic therapy that re-balances pathological neural networks.
Breakthrough designation recognizes devices that represent a breakthrough or offer a reasonable proof of significant advantage over existing approved or cleared alternatives. It also expedites the development and FDA review of new devices with the potential to more effectively treat or diagnose life-threatening or irreversibly debilitating conditions.
FDA recognition of the INBRAIN system as a Breakthrough Device means the company will receive feedback throughout the regulatory process, as well as prioritized review by the agency.