Levee Medical has closed an initial oversubscribed series A financing round, raising a total of $6.6 million.
The funds will be used to advance product development, scale infrastructure, and expand the team to support the development of the company's Voro Urologic Scaffold, a bioresorbable post-prostatectomy implant.
"We are extremely pleased to secure this financing with strong support from our investors. It reflects their confidence in our ability to address a significant unmet need for patients recovering from radical prostatectomy," says Bruce Choi, Founder, and CTO of Levee Medical. "This positive momentum provides the company with a solid foundation as we move towards achieving important development and clinical milestones."
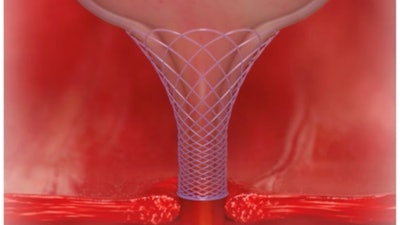
The Voro Urologic Scaffold is a bioresorbable device designed to be placed during the prostatectomy procedure for the treatment of urinary incontinence. It is designed to reduce the stress on the urinary sphincter by managing the geometry of the bladder neck and maintaining urethral length, which is the best predictor of post-op incontinence.
"I strongly believe the Levee technology could significantly decrease the incidence of post-prostatectomy incontinence and vastly change the way both urologists and patients perceive radical prostatectomy," said Jeffrey Dann, M.D., urologist at the Advanced Urology Institute in Palm Coast, FL, and Former Chairman of AUA New Technology Assessment and Coding and Reimbursement Committees. "Providing patients with a cost-effective solution that allows them to return to daily life without the burden of incontinence represents a much-needed breakthrough in the treatment of prostate cancer."